
Applications to the neuroscience PhD program at the university of Montpellier is open
More informations: https://edcbs2.umontpellier.fr/index.html?language=en&page=future_students§ion=PhDcallapplications
Send applications to freddy.jeanneteau@igf.cnrs.fr before mid mai 2024

We recruit postdoc in 2024 for a European project on the metabolomic and epigenomic of the vulnerability to depression and resistance to treatment. Send applications to freddy.jeanneteau@igf.cnrs.fr
10.30.2023: What causes social fears in autism spectrum disorders?
Yann Dromard, Amélie M. Borie, Prabahan Chakraborty, Françoise Muscatelli, Gilles Guillon, Michel G. Desarménien, Freddy Jeanneteau. Disengagement of Somatostatin Neurons From Lateral Septum Circuitry by Oxytocin and Vasopressin Restores Social Fear Extinction and Suppresses Aggression Outbursts in a Prader-Willi Syndrome Model Biological Psychiatry, 2023, ISSN 0006-3223, https://doi.org/10.1016/j.biopsych.2023.10.016.

A genetic modification that can hinder the ability to decode social safety from social fear.
https://www.biologicalpsychiatryjournal.com/article/S0006-3223(23)01661-X/fulltext
2023: “TRKing” neurons into experience-dependent glucocorticoid response severed in Alzheimer’s disease
https://onlinelibrary.wiley.com/doi/10.1111/jne.13235; https://onlinelibrary.wiley.com/doi/10.1111/jne.13203

Jeanneteau F, Meijer OC, Moisan MP. Structural basis of glucocorticoid receptor signaling bias. J Neuroendocrinol. 2023 Feb;35(2):e13203. Jeanneteau F. Stress and the risk of Alzheimer dementia: Can deconstructed engrams be rebuilt? J Neuroendocrinol. 2023 Sep;35(9):e13235.
06.20.2022: The glucocorticoid footprint on the memory engram
The glucocorticoid footprint on the memory engram
Freddy Jeanneteau and Laurence Coutellier. Current opinion on Endocrine and metabolic research. 2022
https://doi.org/10.1016/j.coemr.2022.100378

Revision of the interpretation of the inverted U-shape relation between cortisol level and behavior based on trans-synaptic retrograde signaling.
06.02.2022: Dissociating the beneficial and harmful effects of glucocorticoids to fight Alzheimer’s disease
Through longitudinal monitoring of cognitive and neurophysiological symptoms, we demonstrate that soluble and solid amyloidogenic pathways reorganize functional neuronal connectivity in diametrically opposed ways. The study published in the journal Acta Neuropathologica communication provides new therapeutic avenues.

Dromard Y, Arango-Lievano M, Borie A, Dedin M, Fontanaud P, Torrent J, Garabedian MJ, Ginsberg SD, Jeanneteau F. Loss of glucocorticoid receptor phosphorylation contributes to cognitive and neurocentric damages of the amyloid-β pathway. Acta Neuropathol Commun. 2022 Jun 22;10(1):91. doi: 10.1186/s40478-022-01396-7. PMID: 35733193; PMCID: PMC9219215.
https://actaneurocomms.biomedcentral.com/articles/10.1186/s40478-022-01396-7
01.25.2021. Vasopressin relieves social deficits in an autism mouse model
Oxytocin and vasopressin are intimately linked: The two hormones can communicate with each other’s receptors, says Sue Carter, professor emerita of biology at Indiana University in Bloomington, who was not involved in the study. So the fact that vasopressin seems to show similar effects as oxytocin in a MAGEL2 mouse model is not surprising, she says.
Spectrum News
For many years, vasopressin has been suspected of playing a role in autism spectrum disorder. Also called antidiuretic hormone, vasopressin is produced by certain brain neurons. It regulates various functions such as thirst and blood pressure but also plays a role in social interactions. Already in 2019, an American clinical trial showed that a nasal spray of vasopressin improved certain symptoms in children with autism spectrum disorder. Researchers show that when vasopressin is administered directly into the brain of a mouse model of autism, their social behavior improves. A result which remains to be confirmed in a larger clinical trial.
Borie AM, Dromard Y, Guillon G, Olma A, Manning M, Muscatelli F, Desarménien MG, Jeanneteau F. Correction of vasopressin deficit in the lateral septum ameliorates social deficits of mouse autism model. J Clin Invest. 2021 Jan 19;131(2):e144450. doi: 10.1172/JCI144450. PMID: 33232306; PMCID: PMC7810497. https://www.jci.org/articles/view/144450

11.08.2021: Fitness of synaptic mitochondria is programmable via stress-regulated gene NR4A1
In vivo imaging of dendritic spines and mitochondria, ATP and ROS suggest remarkable plasticity to stress

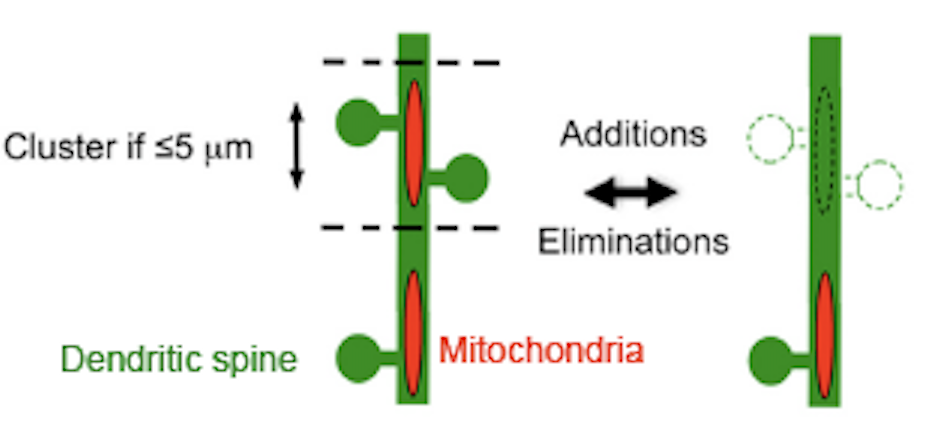
Dromard Y, Arango-Lievano M, Fontanaud P, Tricaud N, Jeanneteau F. Dual imaging of dendritic spines and mitochondria in vivo reveals hotspots of plasticity and metabolic adaptation to stress. Neurobiol Stress. 2021 Sep 21;15:100402. doi: 10.1016/j.ynstr.2021.100402. PMID: 34611532; PMCID: PMC8477201. https://www.sciencedirect.com/science/article/pii/S2352289521001107?via%3Dihub
11.08.2020: Diversity of glucocorticoid response is healthy:
Experience and activity-dependent control of glucocorticoid receptors during the stress response in large-scale brain networks
Damien Huzard, Virginie Rappeneau, Onno C. Meijer, Chadi Touma, Margarita Arango-Lievano, Michael J. Garabedian and Freddy Jeanneteau.

The diversity of actions of the glucocorticoid stress hormones among individuals and within organs, tissues and cells is shaped by age, gender, genetics, metabolism, and the quantity of exposure. However, such factors cannot explain the heterogeneity of responses in the brain within cells of the same lineage, or similar tissue environment, or in the same individual. Here, we argue that the stress response is continuously updated by synchronized neural activity on large-scale brain networks. This occurs at the molecular, cellular and behavioral levels by crosstalk communication between activity-dependent and glucocorticoid signaling pathways, which updates the diversity of responses based on prior experience. Such a Bayesian process determines adaptation to the demands of the body and external world. We propose a framework for understanding how the diversity of glucocorticoid actions throughout brain networks is essential for supporting optimal health, while its disruption may contribute to the pathophysiology of stress-related disorders, such as major depression, and resistance to therapeutic treatments.Keywords: Allostery, epigenetic, plasticity, synapse, stress-related disorders, Bayesian brain
Cerebrovascular research in the lab:
1) Regeneration of the neurogliovascular unit.
Arango-Lievano M, Dromard Y, Fontanaud P, Lafont C, Mollard P, Jeanneteau F. Regeneration of the neurogliovascular unit visualized in vivo by transcranial live-cell imaging. J Neurosci Methods. 2020 Sep 1;343:108808. doi: 10.1016/j.jneumeth.2020.108808 https://www.sciencedirect.com/science/article/abs/pii/S0165027020302314?via%3Dihub
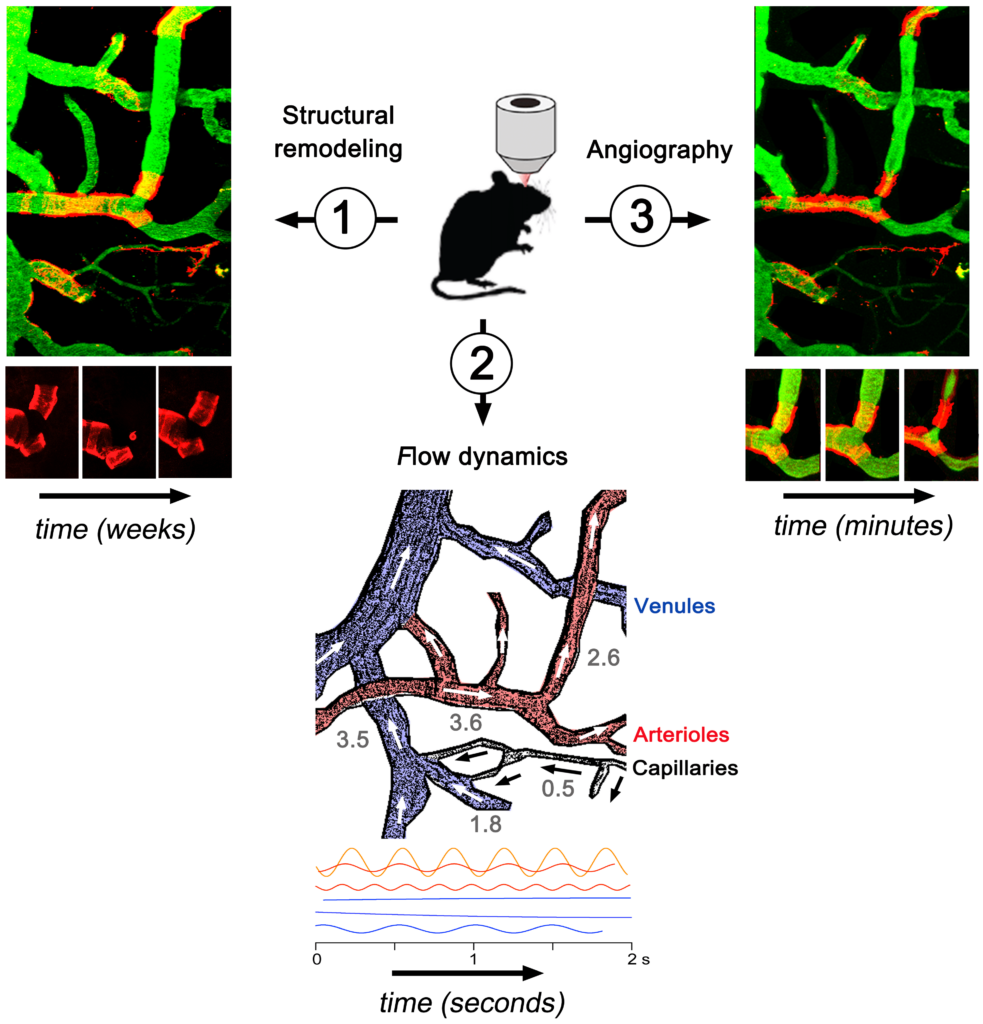
2) Treating pericytosis and cerebrovascular dysfunctions in Epilepsy

Arango-Lievano M, Boussadia B, De Terdonck LDT, Gault C, Fontanaud P, Lafont C, Mollard P, Marchi N, Jeanneteau F. Topographic Reorganization of Cerebrovascular Mural Cells under Seizure Conditions. Cell Rep. 2018 Apr 24;23(4):1045-1059. doi: 10.1016/j.celrep.2018.03.110 https://www.cell.com/cell-reports/fulltext/S2211-1247(18)30486-8?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2211124718304868%3Fshowall%3Dtrue
11.08.2019 Cognitive signaling of stress on the synaptic engram

Arango-Lievano M, Borie AM, Dromard Y, Murat M, Desarmenien MG, Garabedian MJ, Jeanneteau F. Persistence of learning-induced synapses depends on neurotrophic priming of glucocorticoid receptors. Proc Natl Acad Sci U S A. 2019 Jun 25;116(26):13097-13106. doi: 10.1073/pnas.1903203116 https://www.pnas.org/doi/abs/10.1073/pnas.1903203116?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
2019: Cracking the BDNF and glucocorticoid signaling interplay in the brain

antagonists in the mesolimbic areas reversed the consequences
of chronic stress and depressive-like behaviors. Likewise, GR antagonists impeded
stress-induced sensitization of the mesolimbic pathway,
but on the other hand GR agonists prevented behavioral
vulnerability to stress in corticolimbic areas.
Jeanneteau F, Borie A, Chao MV, Garabedian MJ. Bridging the Gap between Brain-Derived Neurotrophic Factor and Glucocorticoid Effects on Brain Networks. Neuroendocrinology. 2019;109(3):277-284. doi: 10.1159/000496392 https://karger.com/nen/article/109/3/277/220609/Bridging-the-Gap-between-Brain-Derived
2015 BDNF uses the glucocorticoid receptor as signaling platform to promote synaptic plasticity of stress

Arango-Lievano M, Lambert WM, Bath KG, Garabedian MJ, Chao MV, Jeanneteau F. Neurotrophic-priming of glucocorticoid receptor signaling is essential for neuronal plasticity to stress and antidepressant treatment. Proc Natl Acad Sci U S A. 2015 Dec 22;112(51):15737-42 https://www.pnas.org/doi/abs/10.1073/pnas.1509045112?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
2016 DUSP1 is a TAU kinases phosphatase

Arango-Lievano M, Peguet C, Catteau M, Parmentier ML, Wu S, Chao MV, Ginsberg SD, Jeanneteau F. Deletion of Neurotrophin Signaling through the Glucocorticoid Receptor Pathway Causes Tau Neuropathology. Sci Rep. 2016 Nov 16;6:37231. doi: 10.1038/srep37231
https://www.nature.com/articles/srep37231
2016 Palmitoylation regulates physical interaction between a GPCR and its signaling effector: The case of the dopamine Drd3 receptor

Arango-Lievano M, Sensoy O, Borie A, Corbani M, Guillon G, Sokoloff P, Weinstein H, Jeanneteau F. A GIPC1-Palmitate Switch Modulates Dopamine Drd3 Receptor Trafficking and Signaling. Mol Cell Biol. 2016 Jan 19;36(6):1019-31. doi: 10.1128/MCB.00916-15
https://www.tandfonline.com/doi/full/10.1128/MCB.00916-15
PORTRAIT du laboratoire sur le site de la Fondation pour la Recherche Médicale (FRM): Lien ICI


INTERVIEW of Freddy for LePoint.fr (FR): ” Les inquiétantes conséquences à long terme du stress précoce“
Overview of our research:
We are studying the systems and neuronal networks implicated in the regulation of behaviors in mice models.
Two-Photon Imaging

Our lab uses a variety of technologies in order to determine the circuitry regulating social behaviors (link), memory formation (link) and …
(Photo by Nicolas Six)
The Live Mouse Tracker
We use the Live Mouse Tracker, an open-source technology, to study social behaviors of groups of mice.
(Photo by Nicolas Six)

Mice house in groups display a complex range of natural and social behaviors.
